Alpha2EQ® Science
What does the science say?
Introduction to the Molecule
Alpha-2-macroglobulin (α2M) is a large glycoprotein, evolutionarily conserved across both invertebrate and vertebrate lines as part of the innate immune system.1 α2M is classified as an acute phase protein, synthesized mainly by hepatocytes, but also by additional cell types, including macrophages and synoviocytes.2,3 The major function of α2M is the non-specific inhibition of all four classes of proteases4, but α2M has many diverse and complex additional functions. α2M binds and regulates the activity of a number of cytokines, binds and regulates the activity of a number of hormones, and has demonstrated regulation of genes.5 While α2M has demonstrated roles principally in fighting disease as part of the innate immune system, the same functions are the basis for utilizing α2M in the presence of inflammatory conditions of the musculoskeletal system.
Mechanism of Protease Inhibition
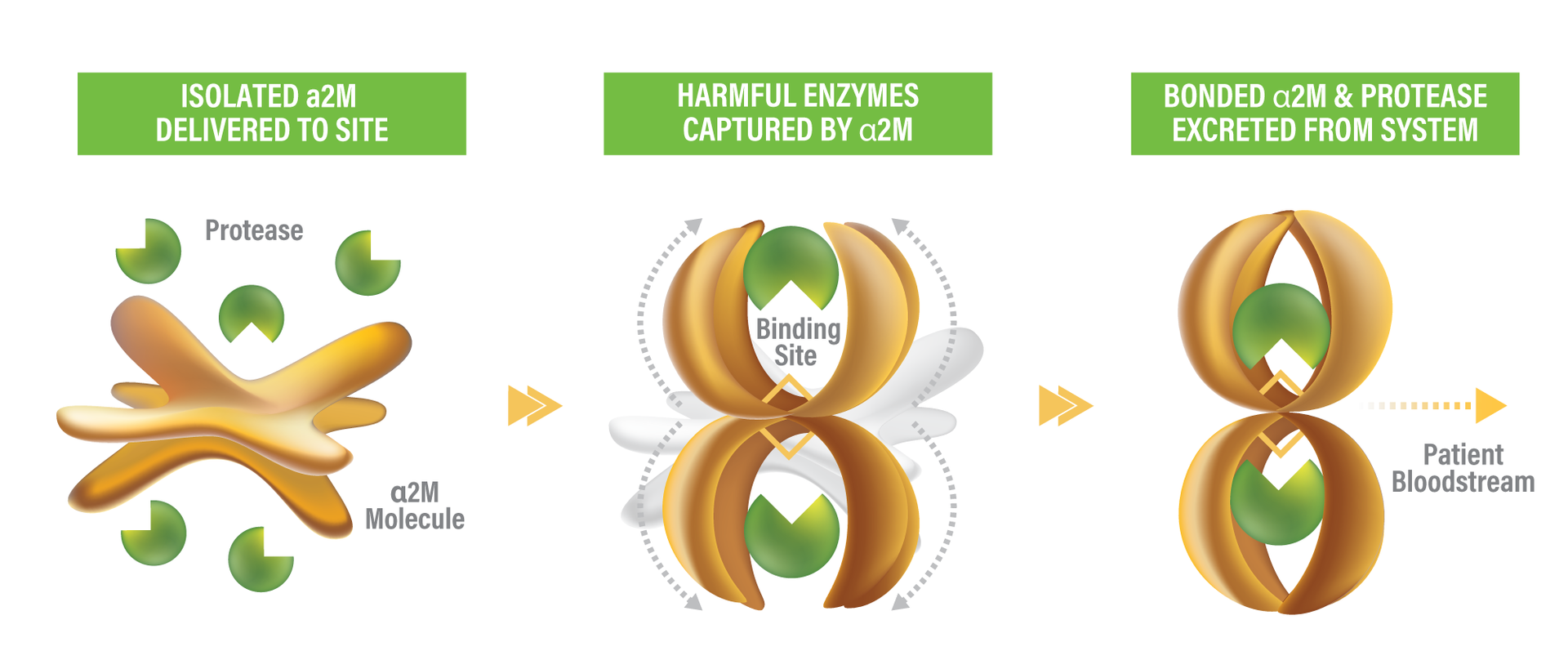
Alpha-2-macroglobulin is unique amongst plasma protease inhibitors because of its ability to inhibit virtually any protease, regardless of the proteases’ specificity or catalytic mechanism.5 α2M in most mammalian species is composed of four identical monomeric subunits, approximately 180k Daltons in size, arranged as a tetramer, or a “dimer of dimers.” The means of α2M protease inhibition has been referred to as the “trapping” mechanism. A short, unique segment of amino acids near the middle of the polypeptide chain acts as a “bait” region, which is vulnerable to cleavage by most proteases. After a protease cleaves the “bait” region, conformational changes in the α2M molecule are triggered— springing the “trap” — resulting in entrapment of the protease molecule.6 Trapping the protease molecule produces two important results: the protease molecule is sterically hindered from accessing its substrate, and the receptor binding site on each α2M monomer is exposed, enabling those molecules containing protease to be bound and cleared via phagocytosis— unbound α2M is not cleared from the site.5
Osteoarthritis
Osteoarthritis is an active response to joint injury resulting from abnormal remodeling of joint tissues driven by a host of inflammatory mediators within the affected joint.7 The extracellular matrix of articular cartilage is composed of proteins and glycoproteins, principally collagen, and several others, including aggrecan and cartilage oligomeric matrix protein (COMP).8,9 The progression of osteoarthritis is driven, at least in part, by upregulation of cartilage matrix degrading proteases, pro-inflammatory cytokines and genes that modulate inflammatory or catabolic processes within the joint.7 The result is loss of articular cartilage caused by extracellular matrix breakdown— the hallmark of arthritis.10 This multi-factorial cascade of events may be amenable to molecular interventions targeting these mediators of joint degradation, but it is unlikely that blocking only one of these catabolic factors would be sufficient to suppress the multiple inflammatory and catabolic factors involved in the progression of osteoarthritis.11

α2M as an Orthobiologic
Alpha-2-macroglobulin is synthesized by synoviocytes and chondrocytes with measurable levels in synovial fluid that are lower than levels in the serum, in part due to the large molecular weight preventing diffusion into the joint. 3 α2M has been demonstrated in the joints of many species, including dogs and horses. 12, 13 While α2M is a negative regulator of the catabolic factors associated with joint trauma and osteoarthritis, it is not present at sufficient levels to suppress all of the catabolic factors in an inflamed joint. Intra-articular supplementation of α2M may provide protection for the joint. 3
Alpha2-macroglobulin is recognized as a protease inhibitor of all four classes of proteases. The matrix metalloproteases (MMP) and the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) classes of proteases are integral to the degradation of articular cartilage. 10, 14 α2M has been shown to be an endogenous inhibitor of matrix metalloproteinases-1, -9, and -13 (collagenases) and MMP-3 (stromelysin). 3, 15 ADAMTS-7 and -12 degrade cartilage oligomeric matrix protein (COMP) and are induced in the cartilage and synovium of arthritic joints. 16 ADAMTS-4 and -5 are aggrecanases that directly result in the loss of aggrecan from the extracellular matrix of articular cartilage in osteoarthritic joints. 17 Studies have demonstrated α2M inhibition of ADAMTS-7 and -12, and ADAMTS-4 and -5 in a concentration dependent manner. 16, 17 The essentially complete inhibition of collagenases and other matrix degrading enzymes by α2M may represent an important protection for articular cartilage. 18
The role of pro-inflammatory cytokines, principally interleukin-1 (IL-1) and tumor necrosis factor α (TNFα), in cartilage degradation is well established. 19 The pleiotropic effects of cytokines can lead to induction of additional cytokines and upregulation of genes. In traumatized joints, IL-1β release can induce the release of several catabolic cytokines and enzymes, including additional IL-1β, TNFα, MMP-3, and MMP-13.3 Treatment of in vitro chondrocytes with α2M results in decreased protein levels of the majority of cartilage catabolic cytokines and enzymes induced by IL-1β.3 α2M has demonstrated the ability to bind to IL-1β, most effectively when bound to a protease, inhibiting its biological effects, and enabling the complex to be cleared via phagocytosis. 5, 20 Similar results have been obtained when investigating the interaction of α2M and TNFα: protease bound α2M binds to TNFα, modulating its effects and facilitating clearance. 21 α2M has been shown to bind to several additional cytokines and growth factors, including, but not limited to, transforming growth factor β (TGF-β) in the synovial fluid of inflamed equine joints, supporting the role of α2M in modulating the effects of TGF-β in inflammatory joint disease. 13 α2M has demonstrated modulation of the effects of several cytokines and growth factors playing integral roles in the cascade of events leading to degradation of articular cartilage. This direct evidence of physical association of cytokines with α2M support its role as an orthobiological response modifier. 2
More recently, alpha2-macroglobulin’s role as a regulator of genes has been discovered. Using an anterior cruciate ligament transection (ACLT) model in rats, Wang et al, demonstrated that supplemental intraarticular α2M enhanced the levels of mRNA for Col2a1 and Acan (anabolic genes), and suppressed the levels of mRNA for MMP3, MMP13, Runx2, and Col10a1 (catabolic genes) when compared to saline, injected controls. 3 These results suggest that α2M has a chondroprotective effect in vivo by decreasing gene expression of catabolic factors, as well as by increasing anabolic gene expression. 3
Summary
Alpha-2-macroglobulin is unique in possessing a multi-modal mechanism of action — non-specific protease inhibition, cytokine and growth factor modulation, and gene regulation. These mechanisms suggest that, in addition to inhibiting protease activity, α2M supplementation beyond endogenous levels may inhibit osteoarthritic cartilage degradation in vivo by decreasing cartilage catabolic and inflammatory factors. 3 α2M may offer a useful therapeutic approach to the management of osteoarthritis by reducing gene expression of MMPs and ADAMTS involved in cartilage matrix degradation and favoring its repair. 19
Alpha2EQ is a unique orthobiologic with many different applications and uses.
Please contact us for more information and to find your local Alpha2EQ expert.
What People Say About Us

